|
私がジャガイモ等で電池を作ろうと思ったのは,インターネットで知り合ったカルフォルニアの科学の先生がPickle
power by Barry Kluger-Bellと いうデンチオタクの記事を送ってくれたのがきっかけです.この記事によると
アルミー銅の電池は1.2Vの電圧が発生するとあるが,私が作った(簡易)アルミ-銅の電池は0.5V位しか発生しない.おまえが
作った電池は何Vかと尋ねたら
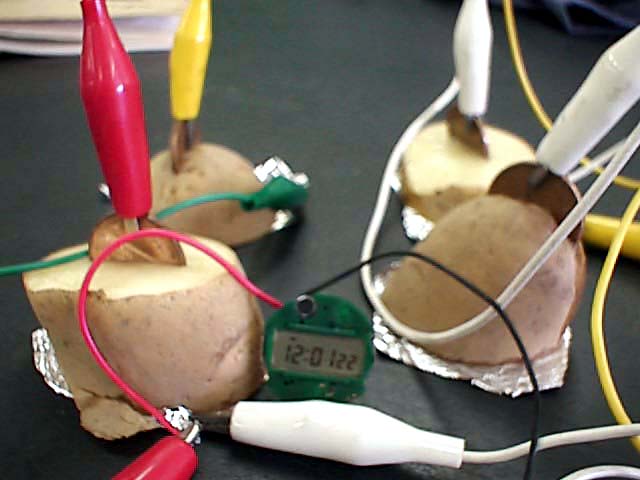
Watashi no koukou no gakusei wa Cu to Zn no
electrodes o tsukaimashita. I don't remember what the voltage
output was. We used two potato cells in series to get enough
voltage to run a Radio Shack "Anywhere Clock" (Radio
Shack part #63-736). Kono chiisai tokei runs on 1.5V button cell.
Gakusei no Jennifer wa shigatsu ni tokei o hajimemashita. Soshite
rokugatsu ni mou ugoite imashita (was still running-correct verb?).
所々に妙な日本語が混じっているのは彼が趣味で日本語を勉強しているせいで す.(笑) 間にはさむ物は電解質水溶液であれば何でもいいわけで,ミカンでも
リンゴでも塩水でもいいはずです.彼も
Last year, Paul's and my students experimented
with * Electrolytes: oranges, lemons, limes, grapefruit, pineapples,
mangoes, potatoes, Coca Cola, tea, salt water, distilled water,
bleach (with Cl), et cetera, et cetera. * Electrodes: Fe nails,
thick Cu wire, galvanized (Zn-coated) nails, Al nails, carbon
"rods" from carpenter's pencils, et cetera, et cetera.
They discovered that the voltage, if any, depended on the electrodes
and not on the electrolyte, and they found that Cu and Zn produced
about 0.9 - 1.0 V with most electrolytes.
なんて書いてきました.この辺からどう自然科学するかが問題なんだが,それ はともかく電池作りは中学ー大学初級に良い教材だ.お金もかからぬし危険も
少なく,時計などを動かせるなどの実用価値もある.少なくとも測定すればお しまいなんて事はない.高校-大学の学生には送ってもらった英文の記事や上
のメールなどを読ませて作らせると良いだろう.
Pickle power by Barry Kluger-Bellという記事には電流計の作
り方が説明されていた.それによると電線を10cm位の輪に卷いて,(これは昔 流の線輪というのがよろしかろう)その線輪を立ててまん中に磁石を糸で吊す.
で,その線輪に電流を流すと磁石が踊る訳.私はこれを作って塩水電池3-4個直列につないでみると当然ながら磁石が踊る.私はこれを見て「これはファラ
デーの世界じゃないか」と思った.コップの中では塩水の化学反応,線輪の空間では電磁気の世界だ.ところで同じ事をナショナルの電池にサンワのディジ
タルテスターを使って「この電池は1.53V」なんてやっても何の感慨も湧かないだろう.これは教育実験を考えるときに大事なことだと思う.
私は地震の前の年の秋頃にファラデーの実験ノートの復刻版を読んでいた.そ の時の読書ノートは地震で紛失しているし,前の事なので記憶も曖昧になって
いるので詳しいことは書けないが,大体の事を書くとファラデーはたいそうな 線輪や電池を作って実験していた.そういった事から考えて彼は電気,磁気力
線は流体か伸び縮みする性質のものと考えていたようだ.実際電池から電磁現 象が発生するので流体的な性質があると考えるのは自然な事だ.
マックスウェルはこの実験ノートを見て電磁場は渦などの流体的な性質やゴム のような性質があると考えて電磁場をrotやdiv等の微分方程式で表したのだ.
しかし実際は渦やゴムのような”物”はなく空間の性質だと言うことになって しまった.方程式で表される性質がそこにあるのだからそれは”実在”すると
いう物理学特有のグロテスクな認識になっている.まぁこんな事を考えながら 塩水電池をいじっていた. |